Abstract
Background Chimeric antigen receptor T (CAR-T) cell therapy has been shown to be efficacious in heavily pre-treated patients with relapsed/refractory (r/r) B cell non-Hodgkin lymphoma (BCL) and provides a potential for durable remissions. Despite the encouraging efficacy, the use of CAR-T therapy is associated with high upfront cost, with published estimates of approximately 450,000 USD (based primarily on Medicare data). However, little is known about reimbursed costs related to CAR-T especially among those with private insurance. Therefore, we examined the costs of care around the time of CAR-T therapy using the Blue Cross Blue Shield (BCBS) Axis database of deidentified administrative claims including commercially insured members across the United States.
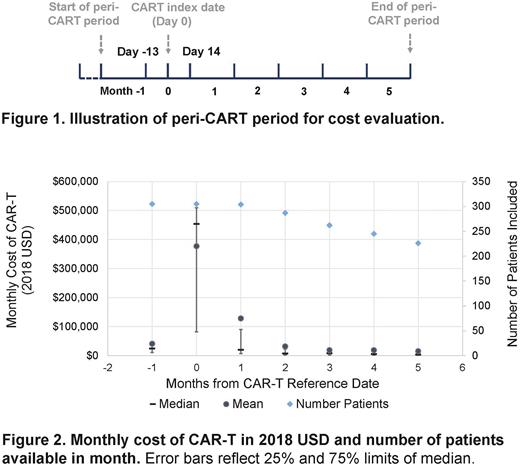
Methods We used deidentified insurance claims from the BCBS Axis database to select individuals with BCL who had claims for CAR-T therapy during 01/2018-06/2021 (Healthcare Common Procedure Coding System [HCPCS] Codes: 0537T, 0538T, 0539T, 0540T, Q2040, Q2041, Q2042, Q2053, Q2054, C9073, C9076). We required the individuals to have continuous enrollment 2 calendar months prior to through at least the calendar month of CAR-T infusion, with age ≥18 years, and to have had claims for diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL), or mantle cell lymphoma (MCL), but not B-cell acute lymphoblastic leukemia. We defined a "peri-CAR-T” period extending from 41 days prior to through 154 days after CAR-T index date, summarized in 28-day intervals (e.g., Month 0 included 13 days prior to through 14 days after CAR-T index, Figure 1).
We estimated the costs of care from allowed amounts on claims (including inpatient, outpatient, professional and associated drug costs in 2018 USD [using CPI-U]) during the peri-CAR-T period and by month. To minimize inclusion of individuals that may have had apheresis or CAR-T therapy manufactured but not administered, our main analytic cohort required a total cost ≥ 250,000 USD reimbursed, a cost substantially lower than the wholesale acquisition costs of CART cell products for BCL and used in a previous cost analysis. We report mean with 95% confidence interval (CI) and median with interquartile range (IQR) for the full sample as well as by BCL sub-types.
Results We identified 473 beneficiaries with at least one claim for CAR-T, with 305 having peri-CAR-T cost ≥ 250,000 USD (median age 58, 68% men) and included in our main analysis. Among the 305 patients, 269 had DLBCL (88%), 6 had FL (2%), and 30 had MCL (10%).
The mean of total costs during the peri-CAR-T period was 618,100 USD (95% CI: 591,800-644,500 USD), with a median of 573,300 USD and IQR of 495,900 to 694,900 USD. Nearly 6% patients had total costs above 1,000,000 USD. The total costs were similar among different types of BCL (mean in USD: 611,900 in DLBCL, 743,100 in FL, 649,100 in MCL). The mean monthly costs were the highest during the month of and following CAR-T infusion (month 0: 377,300 USD; month 1: 128,300 USD), and less than 41,000 USD in any other month (Figure 2). Notably, 168 beneficiaries met the inclusion criteria except having costs < 250,000 USD during the peri-CAR-T period (median age: 57, 62% men, 89% DLBCL, 4% FL, 7% MCL). The mean of total costs in these patients (mean: 110,300 USD, with 75% having total costs ≤ 145,900 USD) was substantially lower than those included in our cost analysis. This population likely represented CAR-T candidates who did not receive the actual CAR-T cell infusion.
Conclusions As the largest real-world cost analysis on CAR-T therapy in the commercially insured population thus far, we report total reimbursed expenditures for CAR-T therapies used in all FDA approved BCL settings. The average expenditure during the peri-CAR-T period was over 600,000 USD, a value substantially greater than what is commonly utilized in cost-effectiveness modeling for CAR-T products. Further, approximately 1/3 of beneficiaries with CAR-T claims had payments <250,000 USD, likely reflecting that a considerable number of individuals do not receive intended CAR-T therapy during real-world practice. As the next step, we will examine average expenditures for CAR-T product, outpatient, and inpatient services, which will be reported at the annual meeting.
Disclosures
Isufi:Epizyme: Membership on an entity's Board of Directors or advisory committees; BEAM Therapeutics: Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite: Speakers Bureau. Foss:Kyowa: Consultancy; Seagen: Consultancy, Speakers Bureau; Astex: Consultancy; Conjupro: Consultancy; Daiichi: Consultancy. Gross:Johnson and Johnson: Research Funding; Genentech: Research Funding; the NCCN Foundation (Astra-Zeneca): Research Funding. Huntington:Genentech: Consultancy; Janssen: Consultancy; Debiopharm Group: Research Funding; TG Therapeutics: Consultancy, Research Funding; Agios: Research Funding; AbbVie: Consultancy; FlatonIron Health: Consultancy; Beigene: Consultancy; AstraZeneca: Consultancy; ADC Therapeutics: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Merck: Consultancy; Seattle Genetics: Consultancy, Honoraria; Tyme: Consultancy; Pharmacyclics: Honoraria; AstraZeneca: Consultancy; Arvinas, Novartis, Servier, Bayer, SeaGen: Consultancy; DTRM Biopharm: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.